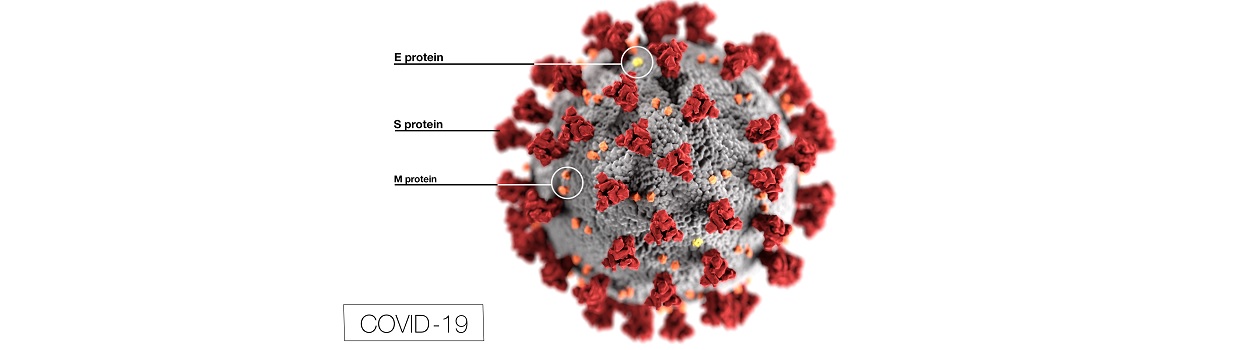
In this course you will learn about SOP on specimen collection, guidelines for screening the patient.
Chapter 1: (PDF Document): WHO Module 5: Differential diagnosis, specimen collection & diagnostic tests. At the end of this module, you will be able to: • Develop a differential diagnosis for patients with severe pneumonia. • Recognize patients with SARI that may have respiratory virus with pandemic potential. • Describe when and what specimens to collect for laboratory diagnosis. • Describe the characteristics of diagnostic tests for respiratory virus infections.
Chapter 2: (PDF Document): WHO Guidance document: Laboratory testing for coronavirus disease (COVID-19) in suspected human cases. Interim guidance issued 19 March 2020.
Chapter 3: (Video, 03 mins): How Coronavirus Test Kits Work: This brief news video by the Wall Street Journal describes the challenge of developing an accurate coronavirus test kit as well some of the ways that testing can produce inaccurate results.
Chapter 4: Guidelines for screening center (NCDC)
Chapter 5: SOP on specimen collection, packaging and transport in ARD: The document from NCDC, provides detailed information about collecting the sample of COVID-19 patient and about its packaging and transportation.
Chapter 6: Specimen collection proforma: The proforma to be filled for collecting samples of positive or suspected COVID-19 patient (NCDC)